Introduction to Atmospheric Ozone
R. Checa-Garcia (CC BY-NC-SA) SCIENCE-BLOG
Atmospheric Chemistry Atmospheric Physics Ozone Stratosphere
Notebook
Still under construction…
Theory of Chapman
It is one of the first, but still useful, theories developed with the objective of explain the production and removing of Ozone on the atmosphere. It proposes a set of chemical reations:
Photochemical formation \[O_{3}+h\nu \longrightarrow O+O\] \[O_{2}+O+M \longrightarrow O_{3} + M\]
Here M is a kind of mediator/catalitic of the chemical reaction, for example \(N_{2}\). And the light involved belongs to the UV should have a wavelenght \(\lambda \leq 242 nm\)
Destruction \[O_{3}+h\nu\longrightarrow O_{2}+O, \qquad \text{with} \qquad \lambda \leq 850 nm\] \[O_{2}+h\nu \longrightarrow O_{2}+O(D), \qquad \text{with} \qquad \lambda \leq 320 nm\]
Producing Oxigen molecule O2 \[O_{3}+O \longrightarrow 2O_{2}\] \[O+O+M \longrightarrow O_{2}+M\]
Here M is also kind of mediator/catalitic of the chemical reaction, for example \(N_{2}\).
The balance between all these equations gives us the estimation of the concentrations.
According with this theory the formation on O3 is larger on those latitudes with higher incoming UV radiation from the sun, and also un the upper-half part of the atmosphere. However also daily and seasonal variations of incoming radiance will have a direct impact on the formation of Ozone.

Ozone Depletion
The Theory of Chapman describes the production of Ozone on the stratosphere but it fails to full describe the ozone depletion that happened mainly during the 70’s and 80’s. Whence, the original proposal was complemented by chemical reactions involving the now commontly named: ozone depleting substances (ODS) in oposition to for example, ozone precursors. Two families of ODS are named chlorine and bromine compounds. In the case of the chlorine (for examples CFCs) it is possible to have now reactions like: \[CFCl_{3}+h\nu \longrightarrow Cl +\, others\]
and \[Cl+O_{3} \longrightarrow ClO +O_{2}, \qquad \text{with} \qquad \lambda \leq 230 nm\]
And we can realize that the previous theory of Chapman is now incomplete. Also similar reactions involving Br, \(NO_{x}\) or \(HO_{x}\) compounds are possible. Obviusly the process is more complex, and for example Cl can react with \(CH_{4}\). Finally note that these chain-reactions are conditioned by the atmospheric transport on the trosposphere and stratosphere, and also scavening processes on the troposphere.
Transport of Ozone
Large-scale diabatic circulation
It is known as Brewer-Dobson circulation, and it consist on two cells, on each hemisphere ascendent on the tropics, descedent on highlatitudes, which a wave driven transport on the extratropics. Accelerations and decelerations of the Brewer-Dobson circulation is nowdays an active field of research.
A direct consquence of the large-scale circulation is a lower total concentration of Ozone in the tropics and higher on mid to high latitudes but with a strong seasonal component. As a consequence, on highlatitudes we expect higher concentrations on Spring on the Northern Hemisphere but the higher concentrations on Souther Hemisphere are found mostly in November to December. This lower total concentration of ozone on the tropics is due to transport as most of the production of Ozone by UV radiance incidence is happing on these regions.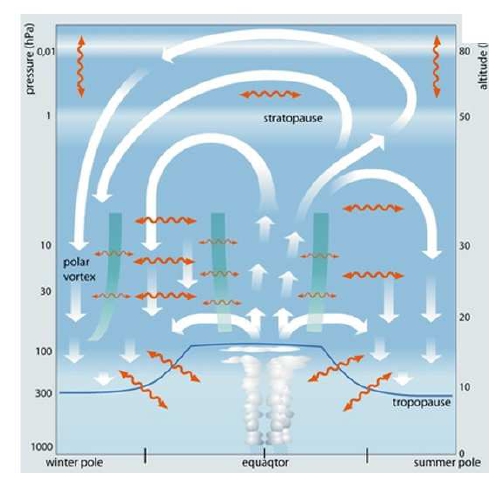
Polar Vortex
However, there is another large scale feature named Polar Vortex which can refers to the tropospheric polar vortex or to the stratospheric polar vortex. Here we are mainly focused on the stratospheric polar vortex which has less extension in terms of latitude. The presence of the stratospheric polar vortex has also a seasonal cycle being stronger in Summer-Autumn on the Southern Hemisphere, for example. In this situation the air masses from the tropics rich in ozone can not penetrate on the polar vortex and ozone depletion is larger with the contribution of the polar stratospheric clouds.
Definitions
Half-Life: it is the time that a species need to decrease its concentration to half of its initial value.